The name already reveals it: If you are working with a rotary evaporator, clearly you want to evaporate something. This process is known in the culinary world mainly as part of the reduction process: Sauces are boiled down to intensify the flavor. All that happens is that the amount of water in the sauce is reduced by cooking, allowing the water to come out as steam. The result is a thickened sauce with an intense flavor. The target product here is not the water vapor, but of course what is left in the pot. However, what still happens in the process is that so-called highly volatile aromas escape with the steam due to the heat and are thus lost. Some flavor compounds are also destroyed by the action of the heat. A good example that illustrates this effect is when you compare the taste of fresh fruit to that of jam that has been cooked down.
But what if you had the option of capturing these highly volatile flavors with water vapor and using them too? Or, what if you could work at lower temperatures that would preserve the flavor but still produce the desired result?
This is exactly where the rotary evaporator steps in!
To understand how this device works, we have to take a deeper insight into the basic principle of evaporation - and don't worry, it's all not as complex as you think!
In physics, evaporation means: A liquid, e.g. water, becomes a gas. It therefore changes its state (aggregate state) from liquid to gaseous. They also say: The substance transitions from the liquid phase to the gas phase.
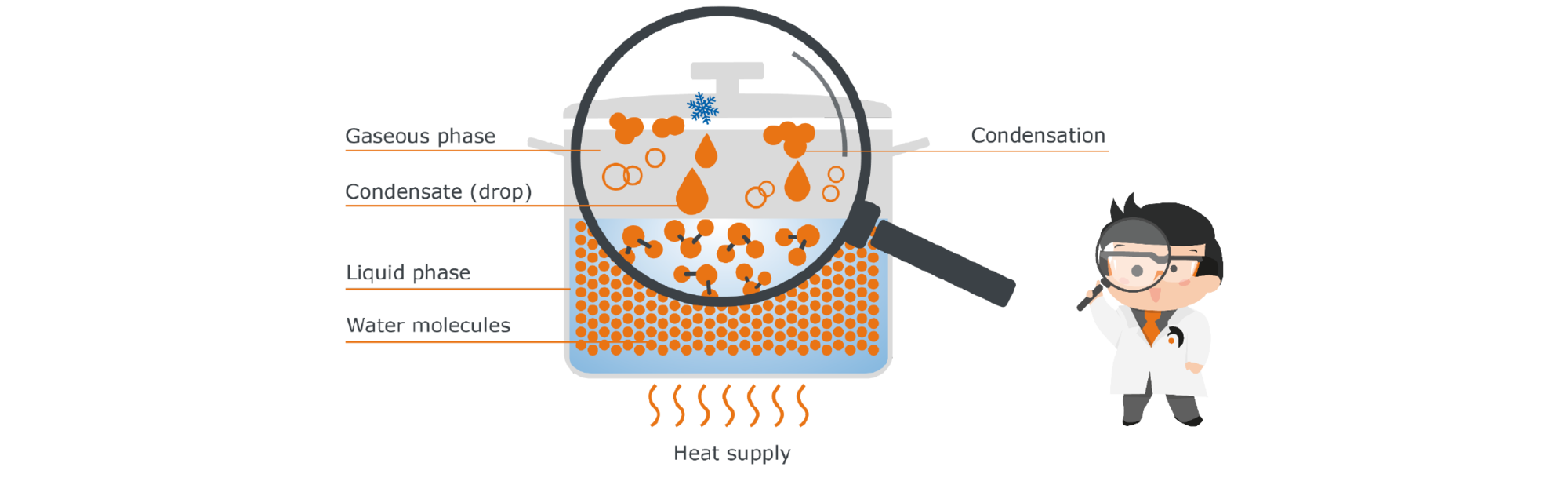
Let's stick with our cooking pot with water as a model: If we simply place the pot on the table, after a long time the water will seemingly disappear. But it is not simply gone: it has evaporated. So, little by little, the water goes from the liquid phase to the gaseous phase, without us contributing anything. To understand why this happens, we have to go to a level that the human eye cannot resolve. Let‘s take a closer look at the water in the pot with the help of Prof. Hei’s molecular magnifier glass:
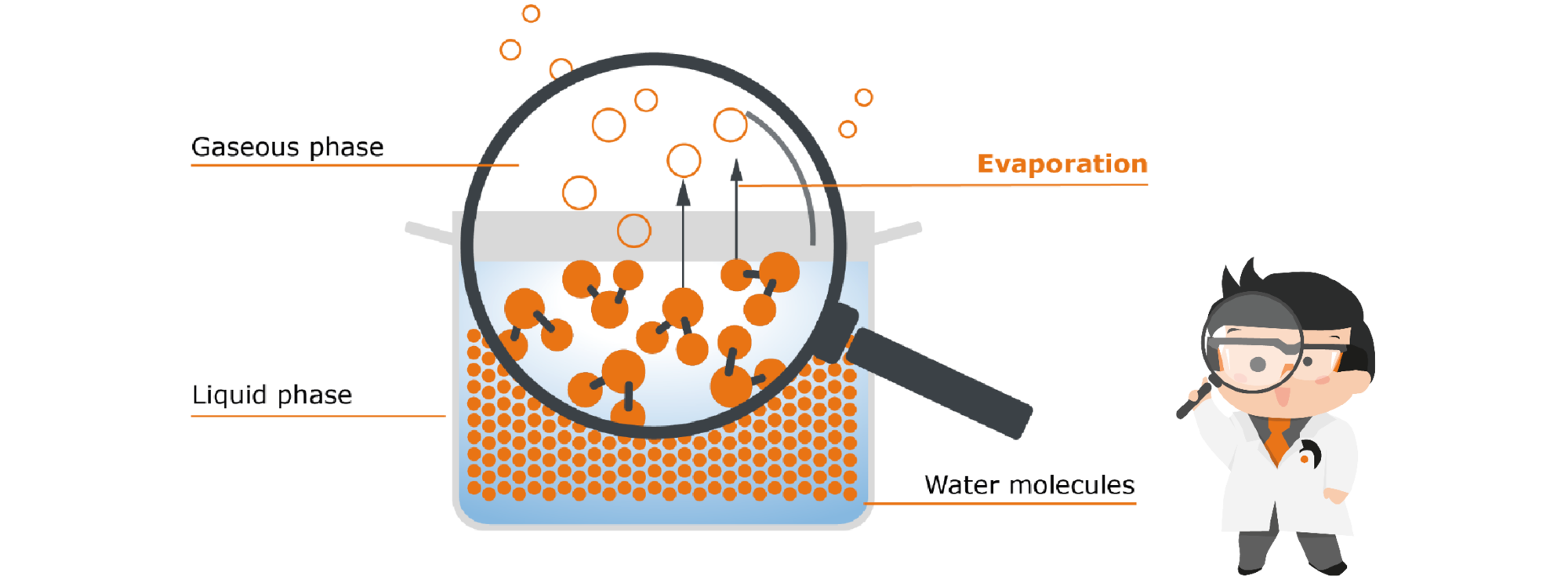
In liquid water, the water molecules are very close to each other. They even hold on to each other by so-called intermolecular bonds, i.e. binding forces between the individual molecules.
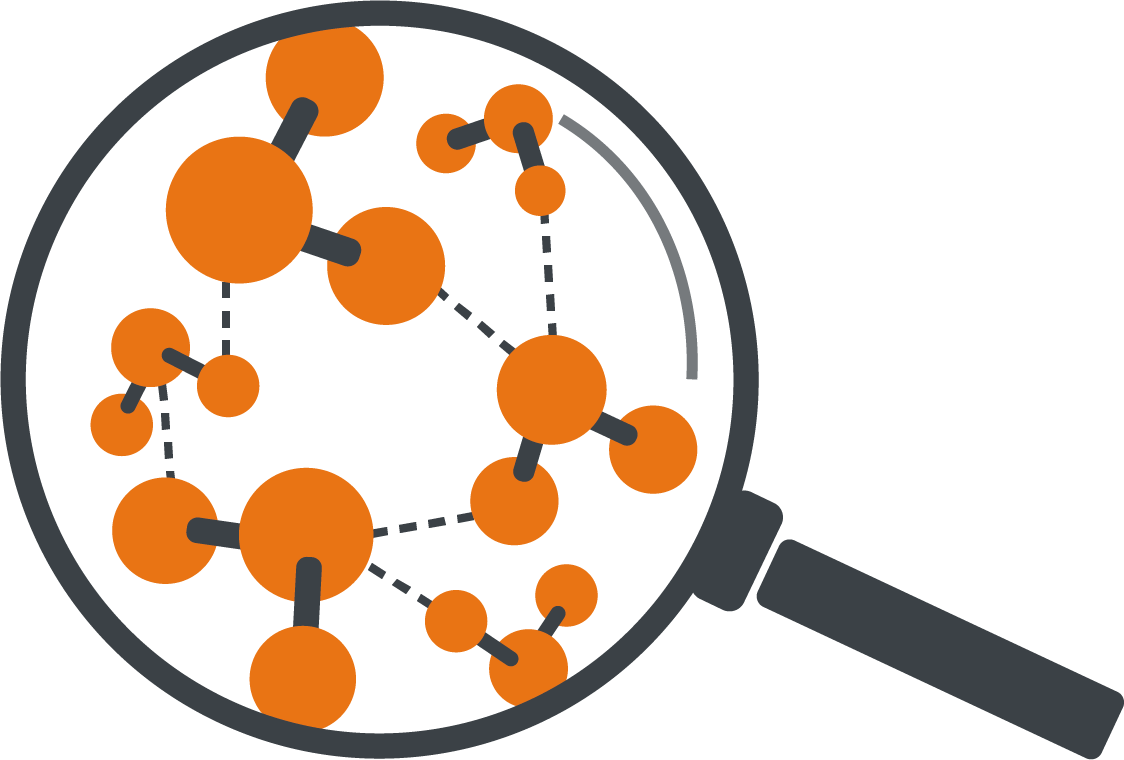
However, they do not simply lie static like marbles in the pot, but are constantly moving. These movements cause the molecules to collide with each other repeatedly, transferring kinetic energy from one molecule to the other. If a single molecule receives a particularly large number of these collisions, it obtains the necessary energy to break the bonds to its partner molecules - in other words, to free itself from the confines of the liquid and rise up out of it. This freedom is desirable for the molecule - understandable, isn‘t it? Who likes to be constricted? That is why molecules always strive to gain as much freedom as possible - they therefore pass from the liquid into the gas phase as soon as they get the necessary energy to do so.
How much energy a substance requires for this varies, depending on the type of substance. Since water or potable alcohol (ethanol) is mainly used in the catering industry, we compare these two liquids: It takes 837 KJ of energy to evaporate one kilogram of alcohol. On the other hand, for one kilogram of water, it is a mere 2,261 KJ. This explains why an evaporation process with water, for example in the production of hydrosoles or reductions / concentrates, is always significantly slower than when working with alcohol.
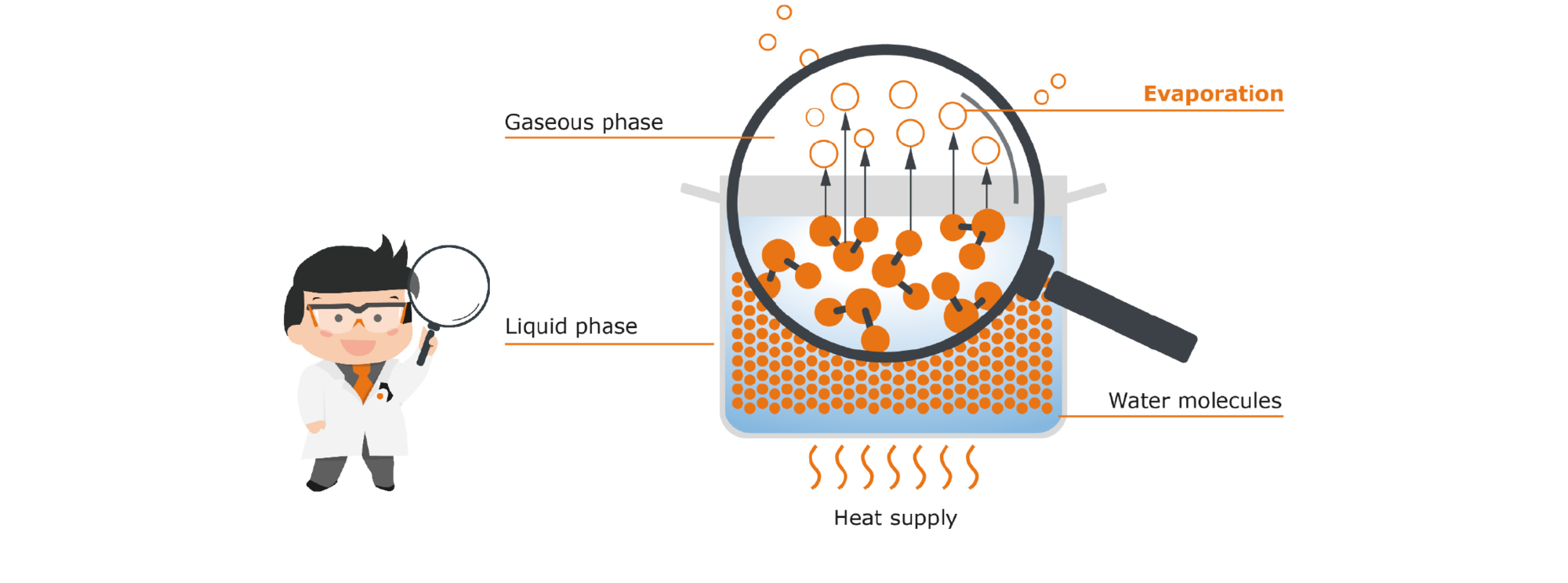
How can this natural evaporation process now be accelerated? The best-known method is to heat the whole thing, which brings us back to the familiar process of reducing: Let’s put our pot with water onto the cooktop: Heat is a form of energy. It causes our molecules to absorb this energy, making them much more agile. There is also an increase in the collisions of the molecules with each other. The result of all this is that the added heat gives many more molecules the energy they need to move from the liquid phase to the gaseous phase. More water therefore evaporates because of the heat supply. The maximum possible amount of vapor is produced when the boiling temperature is reached (i.e. 100 °C for water).
If there are other components in the pot besides water, for example herbs or fruits, the resulting steam contains not only pure water, but also volatile flavors: They are small, distinct molecules that are essential for smell and taste. Together with the low-volatile aromas that remain in the pot, this "molecular cocktail" produces the distinctive odor and flavor profile of the component used. When cooking in the pot, these highly volatile aromas therefore disappear as the steam increases - and with them a significant proportion of the flavor. Often aromas are also sensitive to heat and are destroyed by exposure to excessive heat, which is associated with further loss.
Another way to speed up evaporation is to reduce the pressure. Our pot of water, just like every-thing else, is subject to atmospheric pressure. This varies depending on the geographical altitude, but can be roughly given as 1,000 mbar. This pressure thus counteracts the transition of our water molecules from the liquid to the gas phase. Let us now imagine that we would reduce the atmospheric pressure, e.g. from 1,000 mbar to 50 mbar: Despite the same amount of energy supplied, considerably more of our molecules can now pass from the liquid phase into the gaseous phase.
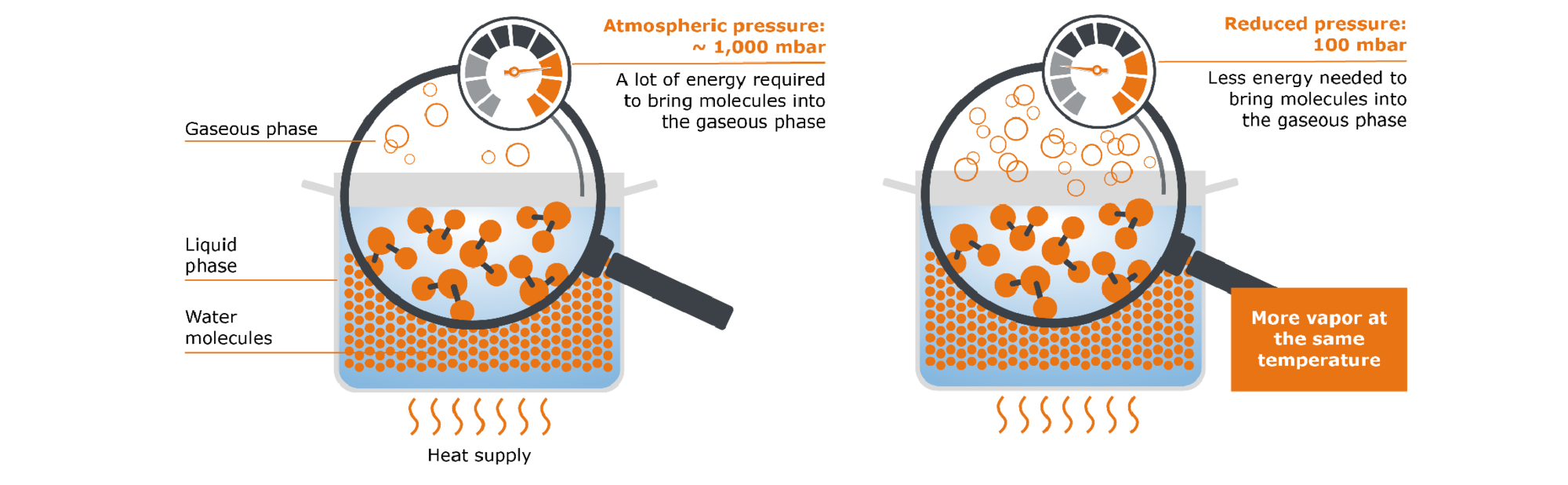
A simple principle can be derived from this: The lower the pressure, the easier a substance can evaporate and the less heat is required for evaporation. The maximum possible amount of vapor can therefore already be generated at lower temperatures (e.g. 50 °C for water at 100 mbar). Here we come to the key point that plays a special role in flavor management with the rotary evaporator: The possibility of working at reduced pressure, i.e. in a vacuum, preserves sensitive aromas that would otherwise be destroyed by heating.
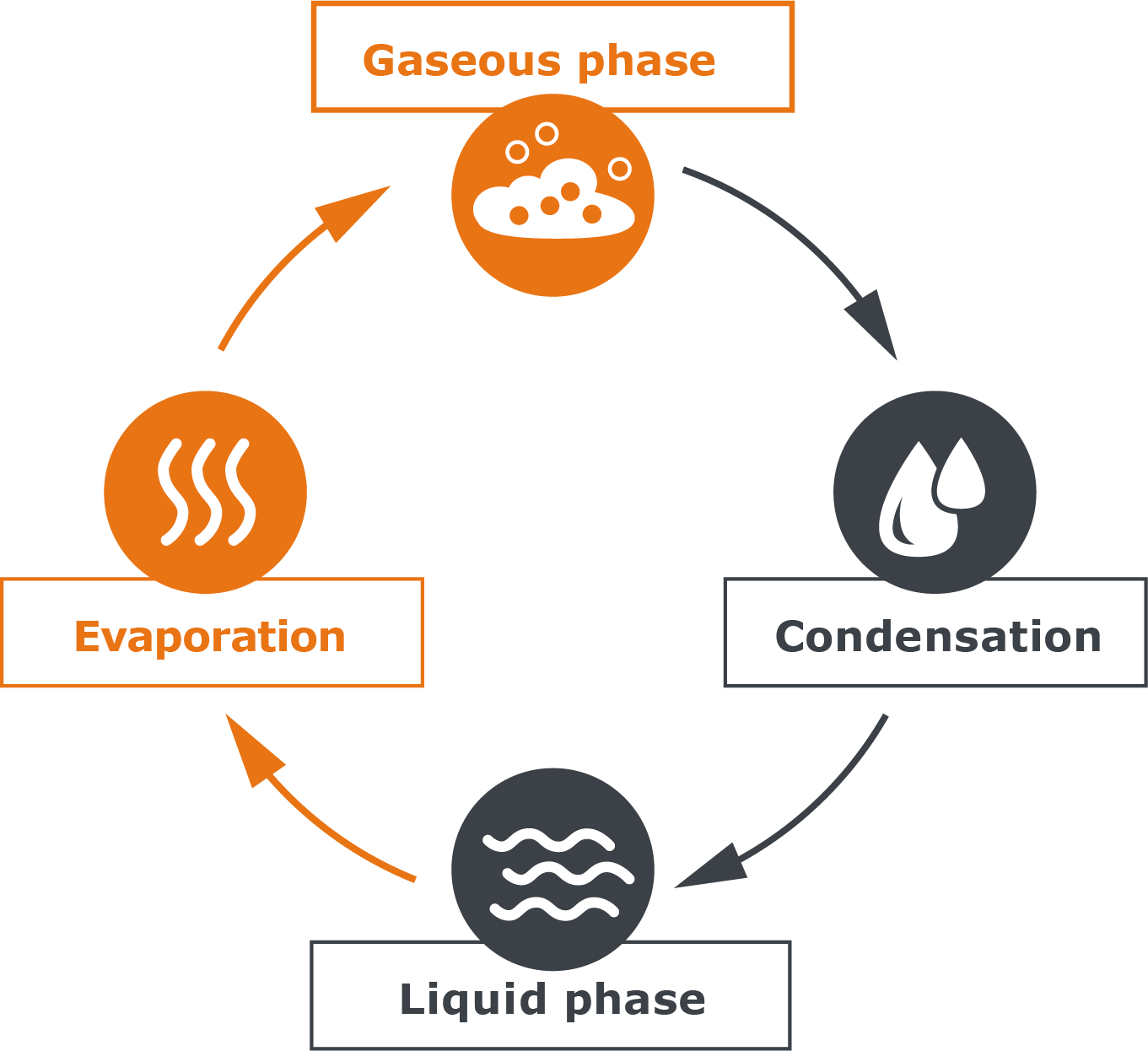
Now that we understand how water turns into steam, we need to look at process in reverse: How does steam turn back into liquid? The answer is: Through condensation. a substance passes from the gaseous phase to the liquid phase. This happens when the energy we have supplied to our liberated water molecules is taken away again. The easiest way to do this is through cold.
To approach the phenomenon of condensation, we again use our example of the pot with water: If we now close the pot with a lid, the steam can no longer simply escape, but it will remain in this closed system of the pot interior. At the same time, the lid of the pot is cooler than the rest: our liberated water molecules therefore collide with this cool barrier and transfer some of their energy to the cooler lid. A temperature equalization takes place. The molecules collect on the cold surface to form droplets. They condense, which is why the condensed water is called ‚condensate‘. At some point, the drops are heavy enough and fall back into the liquid, where the molecules are re-energized and can rise again.
A cycle of evaporation and condensation is formed, which is in equilibrium.
How can this balance between evaporation and condensation be used in a rotary evaporator? If we look at the structure of a rotary evaporator, this can be divided into two areas: The evaporation area, in which a substance is converted from liquid into gaseous phase, and the condensation area, in which gas is condensed back into liquid.
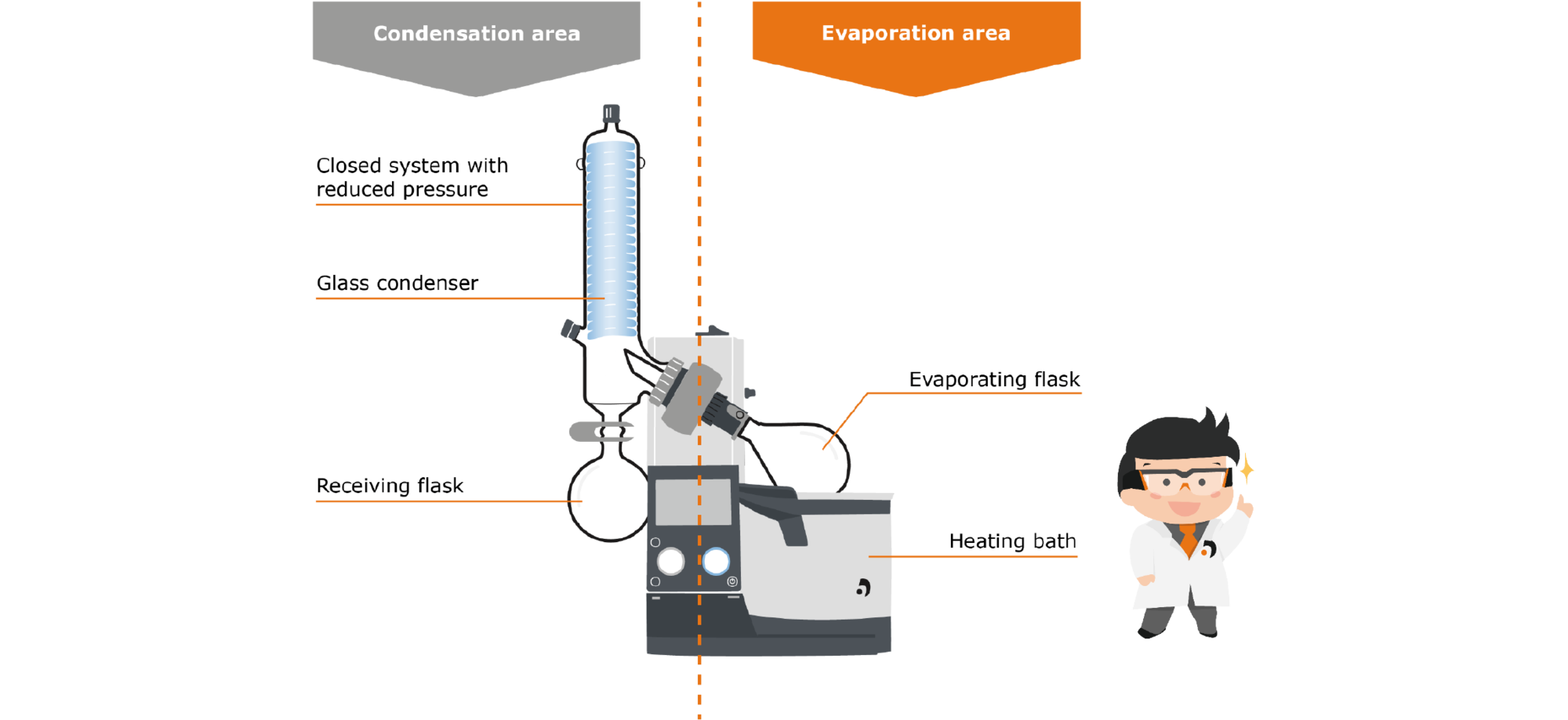
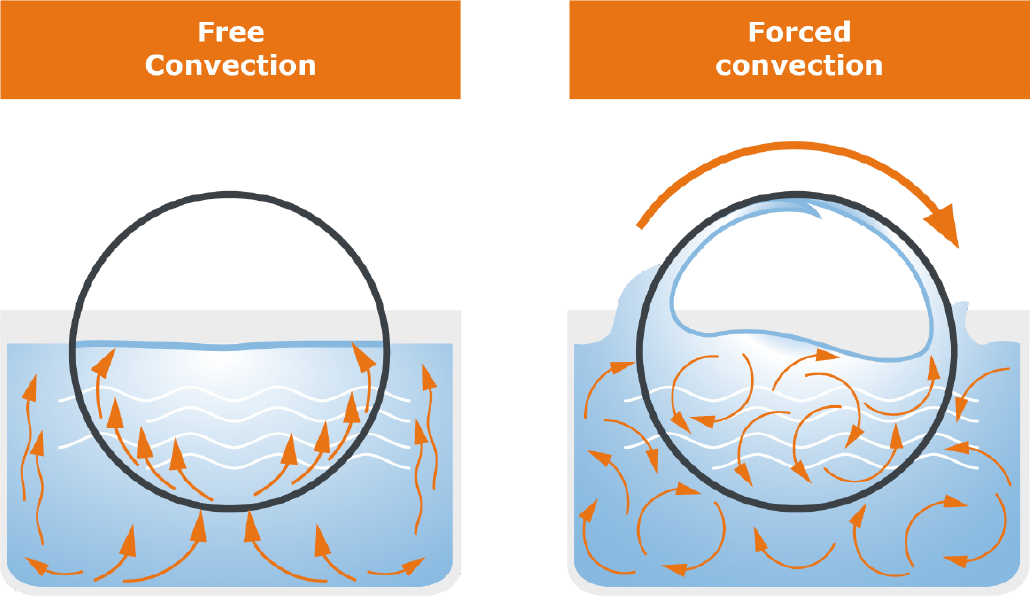
First, let‘s take a closer look at the evaporation area: The area includes the evaporator flask, which contains the liquid medium. This flask is immersed in the heating bath, which supplies the energy necessary for evaporation. The flask is set in rotation by a motor. This has several effects: First, the temperature distribution in the heating bath and inside the flask takes place more uniformly, and second, the rotation significantly increases the surface area available for evaporation. This has a positive effect on the evaporation speed as well as on the formation of bubbles and spills.
The generated steam rises through the steam guide tube into the condensation zone: Here there is a condensercooled with a coolant on which the vapor condenses and drips down into the receiving flask.
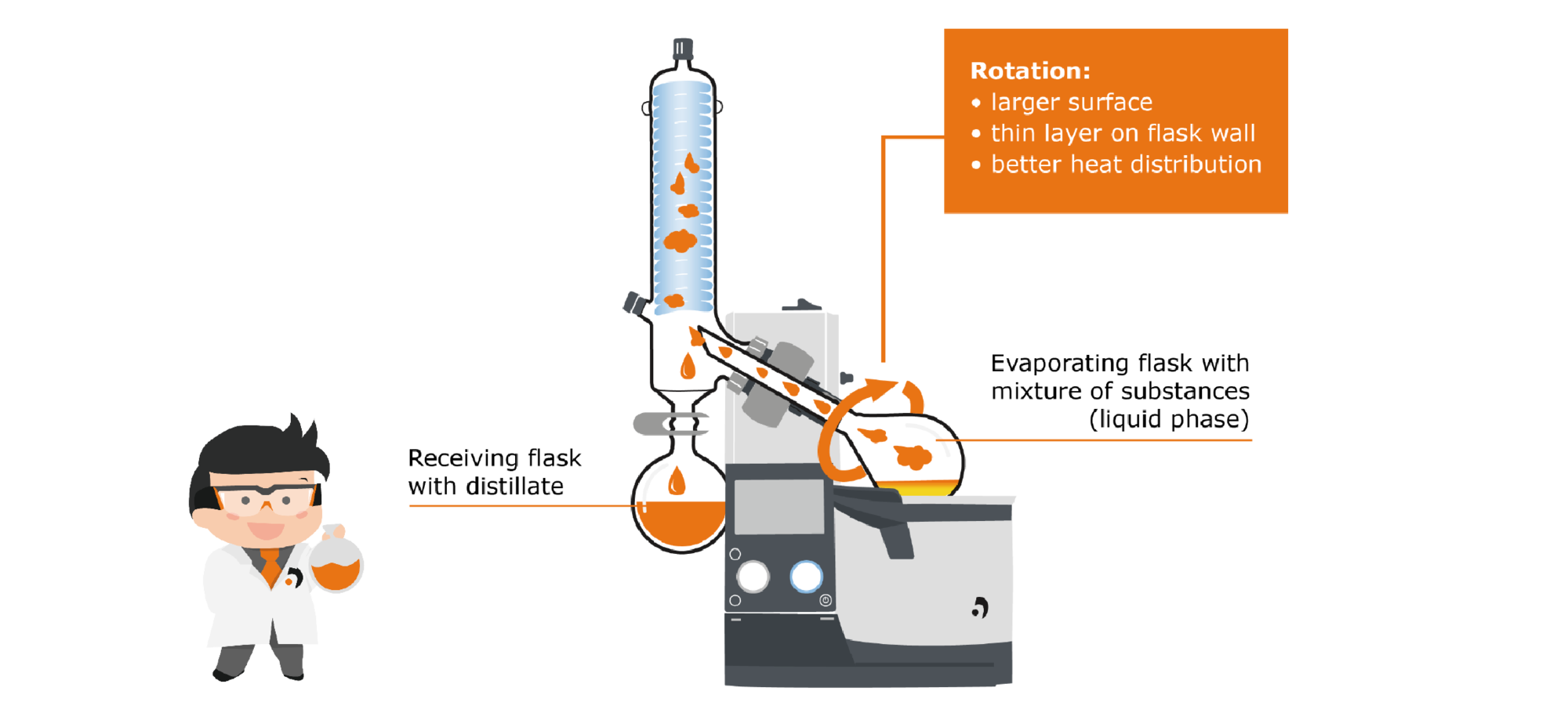
The whole process is carried out in reduced pressure conditions, generated by a vacuum pump to take advantage of the benefits already mentioned above: A lower energy input is necessary to trigger the evaporation and thus the materials are processed more gently. Aromas that would be destroyed by exposure to excessive heat are gently extracted and retained.
In addition, working with a vacuum pump gives one access to a very quickly adjustable parameter to influence the process speed, since no long maintenance times, such as those caused by the inertia of a heating bath, are required. This possibility of fine adjustment also allows aromas to be 'cut off' in a targeted manner and extracted in the way you want them to be.
If you are interested in the low-volatile flavors instead, the rotary evaporator offers the option of reducing sauces, juices or other liquids at low temperatures and thus intensifying their taste.
All in all, this offers countless opportunities to try out flavor management and get creative.